RESOLUTION 004/2025 APPROVES THE GUIDE FOR THE PREPARATION OF PERIODIC SECURITY REPORTS (IPS/PBRER) FOR PANAMA
The Ministry of Health of Panama issued guidelines for the submission of Periodic Safety Reports (IPS/PBRER) by sanitary registration holders in Panama. These reports should include aggregated data, new safety information, and a benefit-risk analysis. The guide seeks to establish a structured framework for monitoring drug safety in the country.
The frequency of reporting varies according to the years of commercialization and the evaluation may generate inspections or modifications in risk management, as follows:
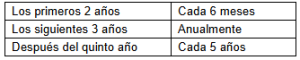
The regulation seeks to strengthen the safety and efficacy of medicines and came into force on February 3, 2025.
For more information, please do not hesitate to contact us.
MarÃa Paula Orsini
Associated
maria.orsini@ariaslaw.com
Issa RodrÃguez See
Associated
issa.rodriguez@ariaslaw.com
The information provided by ARIAS® is presented for informational purposes only. This information is not legal advice and is not intended to create, nor does it constitute, an attorney-client relationship. Readers should not act on this information without seeking advice from professionals in the field.